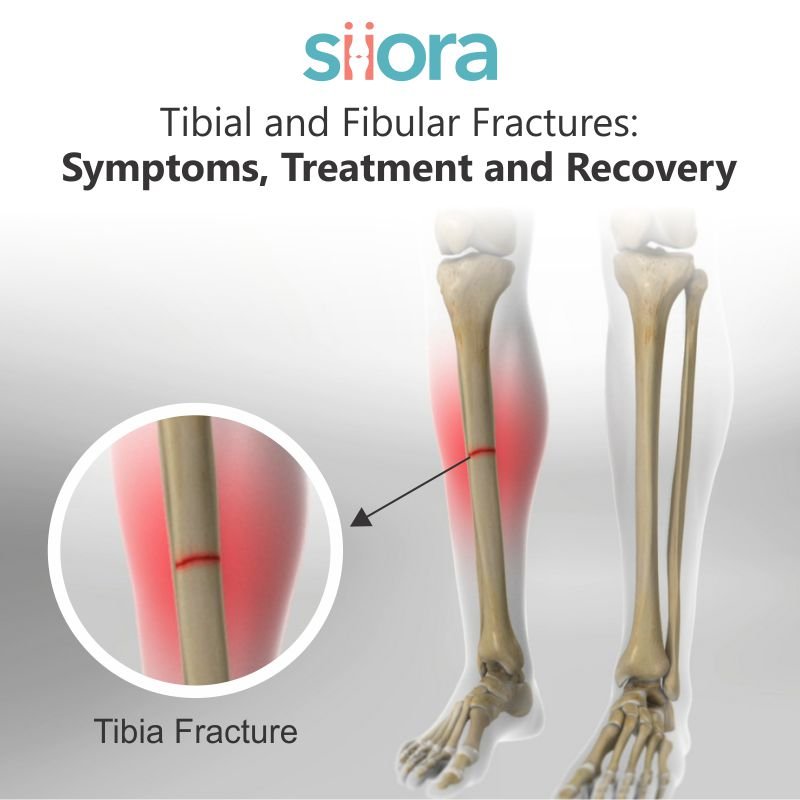
An ideal trauma implant material should be inert, non-toxic to the body, and completely corrosion-proof. It should be inexpensive, simply worked, and mouldable in various shapes without expensive manufacturing techniques. It should have high resistance to fatigue and great strength too. Such a material isn’t available at present.
Stainless Steel
There are at least fifty alloys and grades of alloys known as commercial stainless steel. Only a few are helpful as implant biomaterial in fracture surgery.
Stainless steel designated as ASTM F-55, -56 (grades 316 and 316L) is utilized extensively for fracture fixation implants. Stainless steel of type 316L is an iron-based alloy. Alloying with chromium generates a self-regenerating, protective chromium oxide layer which offers a major protection against corrosion.
The addition of molybdenum reduces the rate of slow, passive dissolution of the chromium oxide layer by up to thousand times. Molybdenum further protects against pitting corrosion. Nickel imparts any further corrosion resistance and enables the production process, while limited quantities of silicon and manganese are added to control some problems related to manufacturing.
The carbon component raises the strength, but its existence in the alloy is undesirable. Under certain conditions created as a results of improper heat treatment, the carbon segregates from the main elements of the alloy, taking with it a considerable amount of chromium in the form of chromium carbide precipitates. Carbides formed at grain boundaries, whereas corrosion selectively happens. Moreover, the carbides degrade the material’s mechanical properties. Mixing of small quantities of titanium or niobium lessens the creation of intergranular carbides by competing for carbon.
Stainless steel of type 316L has a very low permissible level of carbon to diminish this problem. To rise the resistance to fatigue failure, 316L stainless steel can be accessible in a special grade with smaller as well as more widely spaced inclusions. This grade, designated as AISI 316LVM and specified by ASTM F-138, is made by a special method known as ‘vacuum-melting’, which results in a cleaner metal. The strength of 316L may be greatly altered by the method of production, and it may be made extremely ductile (up to 55 percent strain to failure). The alloy selected F-745 is a high strength casting material, whereas 22-13-5 has much more yield strength than 316L for the same treatment method.
Though it is a stiff, strong, and biocompatible material, 316L stainless steel has a slow nevertheless finite corrosion rate. Concerns regarding the long-term effects of nickel ions, still, prevail. Stainless steel is best fitted for short-term implantation within the body as in fracture fixation. Stainless steel is often used because the base materials are cheap, the alloy may be formed using common techniques, as well as its mechanical properties may be controlled over a wide range for ductility and strength. The elastic modulus of stainless steel is approx. 12 times higher than the elastic modulus of cortical bone.
Titanium Alloys
Titanium is the 9th most common element in the crust of earth, where it forms oxidic minerals (ilmenite, rutile). The pure element is very reactive; it’s the sole element that burns in nitrogen. Though, the metal quickly becomes coated with an oxide layer, making it resistant to most chemicals and physiologically inert. Titanium is utilized for making orthopaedic implants in 2 forms: commercially pure and a range of alloys.
Titanium-aluminum-vanadium alloy (ASTM F-136) is generally known as Ti6A14V. This alloy is broadly used to manufacture implants. Impurities such as hydrogen, oxygen and nitrogen tend to make it brittle, which describes why only minimal amounts are acceptable in titanium alloys utilized in surgical implants. ASTM F-136 limits the oxygen concentration to a particularly low level of 0.13%, referred as the ELI (extra low interstitial) grade. Limiting the extent of dissolved oxygen improves the material’s mechanical properties, mainly increasing its fatigue life. Aluminium stabilizes the alpha material’s form while vanadium stabilizes the beta form. Combination of both components forms a two-phase alloy with good strength properties and one that may be heat treated. Ti6A14V ELI is often used for making orthopedic implants.
Commercially pure titanium isn’t a single chemical element however is alloyed by a level of oxygen dissolved into the metal. It also has traces of nitrogen, iron, hydrogen and carbon. The presence of these trace elements effects the mechanical properties of the titanium. International standard ISO 5832/2 defines the extent of permissible impurities.
Titanium has an elastic modulus approx. half that of the cobalt-chromium alloys and stainless steel. The lower stiffness of bone plate made of titanium decreases the severity of stress shielding and cortical osteoporosis. Another benefit of lower stiffness is that a titanium plate is less susceptible to fatigue failure than a stainless-steel plate. The elastic properties of titanium need that contouring be attained by slight over bending; it’s important however, that no metal should be twisted or bent repeatedly at the same location.
The titanium’s modulus of elasticity is still roughly 6 times that of cortical bone. The ductility of titanium alloy is considerably less than that of most stainless steels. Because of this difference a surgeon needs some adaptation of his feel when knowing the optimal amount of torque to be applied to the bone screws. The feel should be acquired prior starting to use these bone screws in clinical practice.
The corrosion resistance of pure titanium is outstanding as a very dense and stable layer can be destroyed mechanically at the time of implantation by instruments such as bending pliers. The passive layer is restored spontaneously, quickly and effectively (re-passivation). In the presence of unstable fixation, the components of titanium of an internal fixation system are exposed to fretting conditions and make metal debris. Such debris causes black or grey coloration of the surrounding tissues. This discoloration, which isn’t a result of corrosion, is harmless. Special surface treatment of the ortho implant lessens such discoloration.